In the world of medical breakthroughs, transforming a simple idea into a life-changing medical device is an inspiring journey. At C3 Medical Device Consulting, we’re privileged to be part of this adventure, helping turn groundbreaking ideas into reality, and making a significant difference in healthcare.
The Beginning: From Dream to Design
Every medical device starts with a simple idea, often born from a desire to fill a gap in healthcare. Picture a healthcare professional or a patient facing a challenge, and then imagine the lightbulb moment when a solution is conceived. This is where the magic starts. It’s not just about having cutting-edge technology but about envisioning a future where healthcare is more accessible, effective, and patient-centered.
Bringing Ideas to Life: Design Meets Development
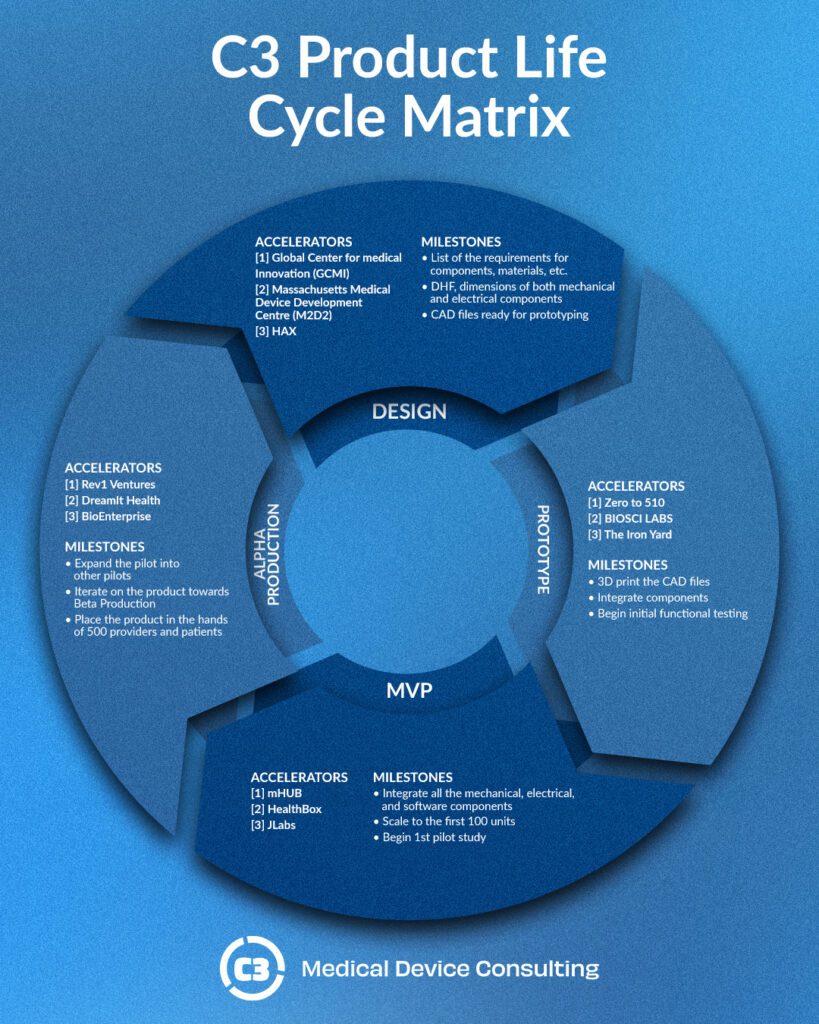
With a vision in place, the next step is turning these ideas into tangible designs and prototypes. This is where creativity meets practicality. Engineers, healthcare professionals, and designers come together, brainstorming and sketching, until a prototype starts to take shape. During this phase, organizations like the Global Center for Medical Innovation (GCMI) and the Massachusetts Medical Device Development Center (M2D2) provide crucial support. They guide teams through the essentials: listing the requirements for components and materials, and preparing CAD files for prototyping. It’s like gathering all the ingredients and the recipe before baking the perfect cake.
Testing and Approval: Ensuring Safety and Efficacy
Before any device can make its debut in the healthcare world, it must pass rigorous testing and regulatory approval. This step is all about safety and making sure the device does what it’s supposed to do without causing harm. Advanced manufacturing techniques, such as electrical prototyping, play a significant role here, allowing for precision and innovation while adhering to strict safety standards.
Launching into the World: Market Introduction
Once a device has cleared all regulatory hurdles, it’s ready to be introduced to the world. This is where strategic marketing and building distribution networks come into play. Accelerators like Zero to 510 and BIOSCI LABS help with transitioning from a working prototype to a product that’s ready for the market, supporting steps such as integrating components and starting functional tests. Imagine seeing your device being used in real-world settings for the first time, making a difference in patients’ lives.
Continuing the Journey: Lifecycle Management
Even after a device hits the market, the journey doesn’t stop. Continuous monitoring, updates, and improvements ensure that the device remains relevant and effective. This phase is about learning from real-world use and making the necessary tweaks to better meet the needs of healthcare providers and patients. Accelerators like Rev1 Ventures and DreamIt Health are key in expanding pilots and iterating towards improved versions, aiming to reach more providers and patients with each iteration.
The Impact: Changing Lives
Looking back on the path from an initial idea to a medical device making an impact, it’s a testament to the power of collaboration, innovation, and a relentless focus on improving patient care. At C3 Medical Device Consulting, we’re honored to guide and witness these transformations, helping turn visionary concepts into devices that enhance healthcare for everyone.