
More than 600 million people are affected by asthma and COPD. The nebulizers created by Wellinks help relieve symptoms for those with these conditions.
When Wellinks switched manufacturers, they lost access to important information, which required our team to start the project from scratch. In a very short time frame, we met all customer goals, effectively reproduced the functionality of the previous design while adding innovative new features and wireless technology.
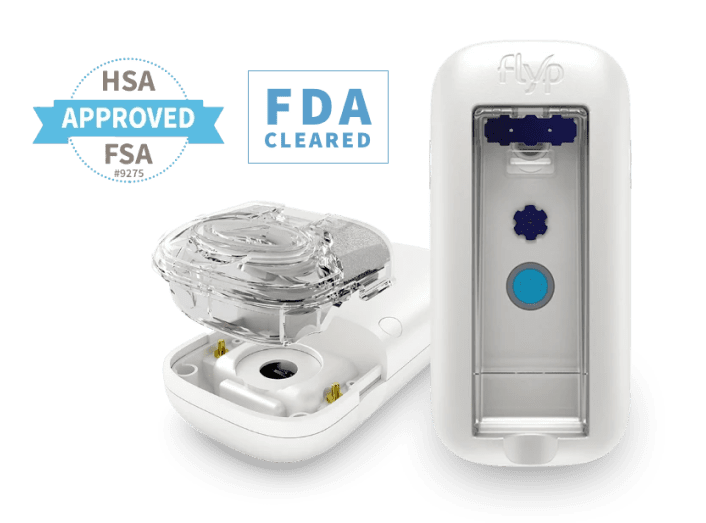
We helped Wellinks reimagine their existing design to produce a portable nebulizer with added features for those who use it.

With no information from the existing manufacturer about the nebulizer, we started this project from scratch on an aggressive deadline.
For user ease, we improved the quality and enhanced the features of the previous design but ensured its appearance and functionality remained the same.
Now, the Wellinks nebulizer boasts wireless capabilities, an electrical hardware redesign, and a firmware redesign. We also developed the back end of the mobile app to integrate with their third-party mobile app team.
While a complete redesign and effective reverse engineering were required, we maintained the original stringent schedule and stayed within the pre-existing FDA certification while making the product even better than before.

Lorem ipsum dolor
Need some help getting your project across the finish line like WellLinks?
Request our FREE design review to ensure your device meets crucial requirements and successfully makes it to market.







Take the guesswork out of the equation with our budgeting calculator and empower yourself to focus on what really matters:
Bringing innovation to life.

The transition to a new manufacturer can present significant challenges for companies like Wellinks, particularly when vital information from the previous manufacturer is lost. This disruption necessitates a comprehensive reassessment of product design and development processes to ensure continuity in quality and functionality.
In Wellinks' case, the lack of access to crucial design data required the team to start the project from scratch. This situation underscores the importance of thorough documentation and communication with manufacturers to mitigate risks associated with such transitions.
The redesigned Wellinks nebulizer introduces a range of innovative features aimed at enhancing user experience and functionality. Key improvements include wireless capabilities that allow users to monitor their device's performance and receive real-time updates via a mobile app.
Moreover, the integration of advanced electrical hardware and firmware improvements not only boosts the device's efficiency but also ensures compliance with FDA regulations. These enhancements reflect Wellinks' commitment to delivering cutting-edge medical technology that prioritizes user needs.
Ensuring compliance with regulatory standards is crucial when redesigning medical devices. Wellinks faced the challenge of maintaining FDA certification while implementing significant changes to their nebulizer's design and functionality.
By adhering to stringent regulatory requirements throughout the redesign process, Wellinks successfully enhanced the product without compromising safety or effectiveness. This balance between innovation and compliance is vital for gaining consumer trust and achieving market success.
Looking ahead, Wellinks aims to expand its product offerings and further innovate within the nebulizer market. The company is committed to leveraging user feedback and technological advancements to enhance the treatment experience for asthma and COPD patients.
As the healthcare landscape evolves, Wellinks plans to explore additional features such as telehealth integration and personalized treatment options, positioning itself as a leader in respiratory care solutions. This forward-thinking approach not only addresses current patient needs but also anticipates future demands in the medical device industry.